Decanol
(
n-Decyl Alcohol)

—C
10H
22O—
158.28—A clear, viscous liquid. Specific gravity: about 0.83 at 20

. Solidifies at about 6.5

. Insoluble in water; soluble in alcohol and in ether.
Assay—
When examined by gas-liquid chromatography, using suitable gas chromatographic apparatus and conditions, it shows a purity of not less than 99%.
Decyl Sodium Sulfate,

C
10H
21NaO
4S—
260.33—White, crystalline solid.
Assay—
Transfer about 1 g, accurately weighed, to a suitable, tared crucible, moisten with a few drops of sulfuric acid, and ignite gently to constant weight. Each mg of residue is equivalent to 3.662 mg of C10H21NaO4S. Not less than 95% is found.
Deoxyadenosine Triphosphate,

C
10H
16N
5O
12P
3—
491.18


[
1927-31-7]—Use a suitable grade.
Deoxycytidine Triphosphate,

C
9H
16N
3O
13P
3—
467.16


[
2056-98-6]—Use a suitable grade.
Deoxyguanosine Triphosphate,

C
10H
16N
5O
13P
3—
507.18


[
2564-35-4]—Use a suitable grade.
Deoxyribonucleic Acid Polymerase—
Thermostable, recombinant DNA polymerase. Use a suitable grade.
Deoxythymidine Triphosphate,

C
10H
17N
2O
14P
3—
482.17—Use a suitable grade.
Deuterium Oxide,

D
2O—
20.032—Use a suitable grade having a minimum isotopic purity of 99.8 atom % of deuterium.
Deuterochloroform,

CDCl
3—
120.38—Use a suitable grade.
Devarda's Alloy
(Devarda's Metal)
—A gray powder composed of 50 parts of copper, 45 parts of aluminum, and 5 parts of zinc.
Dextran, High Molecular Weight—
A dextran molecular weight standard having a weight-average molecular weight,
MW, of 1 to 2 × 10
6 Da and a weight-average molecular weight to number-average molecular weight ratio,
MW /
MN, of 1.0 to 1.8.
Dextrin,

(C
6H
10O
5)
n·
xH
2O—A white amorphous powder. Slowly soluble in cold water; more readily soluble in hot water; insoluble in alcohol.
Insoluble matter—
Boil 1 g with 30 mL of water in a small flask: the solution is colorless and clear, or not more than opalescent.
Loss on drying  731
731 —
—
Dry it at 105

to constant weight: it loses not more than 10.0% of its weight.
Residue on ignition (Reagent test)—
Ignite 1 g with 0.5 mL of sulfuric acid: the residue weighs not more than 5 mg (0.5%).
Chloride (Reagent test)—
Dissolve 3 g in 75 mL of boiling water, cool, dilute with water to 75 mL, and filter if necessary. To 25 mL of the filtrate add 2 mL of nitric acid and 1 mL of
silver nitrate TS, and allow to stand for 5 minutes: any turbidity produced is not greater than that of a control containing 0.02 mg of added Cl (0.002%).
Sulfate (Reagent test, Method I)—
To a 25-mL portion of the filtrate from the preceding test add 0.5 mL of diluted hydrochloric acid and 2 mL of
barium chloride TS, and allow to stand for 10 minutes: any turbidity produced is not greater than that of a control containing 0.2 mg of added SO
4 (0.02%).
Alcohol-soluble substances—
Boil 1 g with 20 mL of alcohol for 5 minutes under a reflux condenser, and filter while hot. Evaporate 10 mL of the filtrate on a steam bath, and dry at 105

: the residue weighs not more than 5 mg (1%).
Reducing sugars—
Shake 2 g with 100 mL of water for 10 minutes, and filter until clear. To 50 mL of the filtrate add 50 mL of
alkaline cupric tartrate TS, and boil for 3 minutes. Filter through a tared filtering crucible, wash with water, then with alcohol, and finally with ether, and dry at 105

for 2 hours: the precipitate of cuprous oxide weighs not more than 115 mg (corresponding to about 5% of reducing sugars as dextrose).
Dextro Calcium Pantothenate
—Use Calcium Pantothenate (USP monograph).
Dextrose, Anhydrous,

C
6H
12O
6—
180.16—Use ACS reagent grade
D-Glucose, Anhydrous.
Diacetyl
—See 2,3-Butanedione .
3,3¢-Diaminobenzidine Hydrochloride,

(NH
2)
2C
6H
3C
6H
3(NH
2)
2·4HCl—
360.11—White to yellowish–tan (occasionally purple), needle-shaped crystals. Soluble in water. Stable in organic solvents but unstable in aqueous solution at room temperature. Store aqueous solutions in a refrigerator.
Insoluble matter—
Dissolve 2 g in 100 mL of water, without heating, and filter immediately: the insoluble residue does not exceed 1 mg (0.05%).
Residue on ignition (Reagent test):

not more than 1 mg, from 2 g (0.05%).
Suitability test for detection of selenium—
Dissolve 1.633 g of selenious acid (H2SeO3) in water, and dilute with water to 1 L. Dilute 10 mL of this solution with water to 1 liter, to make a solution containing 0.010 mg of Se per mL. Place 1 mL of the resulting solution in a 100-mL beaker, add 2 mL of formic acid solution (1 in 7), and dilute with water to 50 mL. Add 2 mL of 3,3¢-diaminobenzidine hydrochloride solution (1 in 200), and allow to stand for 30 to 50 minutes. Adjust with 6 N ammonium hydroxide to a pH between 6 and 7. Transfer to a 125-mL separator, add 10.0 mL of toluene, and shake vigorously for 30 seconds: a distinct yellow color is produced in the toluene layer. A blank containing diaminobenzidine hydrochloride but no selenium standard, treated in the same manner, shows no color in the toluene layer.
2,3-Diaminonaphthalene,

C
10H
10N
2—
158.20—Use a suitable grade.
Diatomaceous Earth, Flux-Calcined
—Use a suitable grade.
[NOTE—A suitable grade is “Chromosorb W, AW-DMCS,” available from Alltech,
www.alltechweb.com.
]
Diatomaceous Earth, Silanized
—Use a suitable grade.
[NOTE—Suitable grades are available commercially as “Anachrome Q,” “Gas-Chrom Q,” and “Varaport 30.”]
Diatomaceous Silica, Calcined
—A form of silica (SiO
2) consisting of fused frustules and fragments of diatoms. It is an amorphous, fine, light pink or white powder. Insoluble in water, in acids, and in dilute solutions of alkali hydroxides.
Loss on ignition—
Accurately weigh about 4 g, and ignite to constant weight: it loses not more than 10.0% of its weight.
Organic impurities—
It does not darken appreciably upon ignition.
Loss on drying  731
731 —
—
Dry it at 110

for 2 hours: it loses not more than 2.0% of its weight.
[NOTE—Suitable grades are “Chromosorb P” and “Chromosorb W,” available from Alltech,
www.alltechweb.com.
]
2,6-Dibromoquinone-chlorimide
(
2,
6-Dibromo-N-chloro-p-benzoquinone Imine; DBQ Reagent),

O:C
6H
2Br
2:NCl—
299.35—A yellow, crystalline powder. Insoluble in water; soluble in alcohol and in dilute alkali hydroxide solutions.
Solubility in alcohol—
A solution of 100 mg in 10 mL of alcohol is not more than faintly turbid.
Residue on ignition (Reagent test)—
Ignite 500 mg with 0.5 mL of sulfuric acid: the residue weighs not more than 1 mg (0.2%).
Sensitiveness—
To 10 mL of a water solution containing 0.01 mg of phenol add 0.3 mL of a sodium borate buffer (made by dissolving 2.84 g of crystallized sodium borate in 90 mL of warm water, adding 8.2 mL of 1 N sodium hydroxide and diluting with water to 100 mL) and 0.1 mL of a solution of 10 mg of the test specimen in 20 mL of alcohol: a distinct blue color develops within 10 minutes.
Dibutylamine,

C
8H
19N—
129.24—Colorless liquid.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 200

; the detector temperature is maintained at 300

; and the column temperature is maintained at 100

and programmed to rise 10

per minute to 200

. The area of the C
8H
19N peak is not less than 99% of the total peak area.
Dibutylammonium Phosphate—
Use a suitable grade.
[NOTE—A suitable grade is available as PIC Reagent D4 from Waters Corporation,
www.waters.com.]
Dibutyl Phthalate,

C
16H
22O
4—
278.34—Clear, colorless liquid.
Assay—
Accurately weigh about 2 g into a suitable flask, add 25.0 mL of 1 N sodium hydroxide and 30 mL of isopropyl alcohol, and mix. Digest the mixture at a temperature near boiling for 30 minutes, then cool in a water bath to room temperature. Add phenolphthalein TS, and titrate with 1 N sulfuric acid VS to the disappearance of the pink color. Perform a complete blank determination, and make any necessary correction. Each mL of 1 N sulfuric acid consumed is equivalent to 139.2 mg of C16H22O4. Not less than 98% is found.
Acid content—
Accurately weigh about 10 g, and dissolve in 100 mL of an alcohol-ether mixture (1:1). Add
phenolphthalein TS, and titrate immediately with 0.05 N alcoholic potassium hydroxide VS. Each mL of 0.05 N alcoholic potassium hydroxide is equivalent to 4.15 mg of phthalic acid: not more than 0.02% is found.
Dichloroacetic Acid,

C
2H
2Cl
2O
2—
128.9


[
79-43-6]—Colorless liquid. Miscible with water, with alcohol, and with ether. Use a suitable grade.
2,5-Dichloroaniline,

Cl
2C
6H
3NH
2—
162.02—White, needle-like crystals. Slightly soluble in water; soluble in alcohol and in ether.
2,6-Dichloroaniline,

C
6H
5Cl
2N—
162.02—Off-white powder.
Change to read:
o-Dichlorobenzene,

C
6H
4Cl
2—
147.00—Clear liquid, having a light yellowish-brown tint (about APHA 20).

 USP29
USP29 Practically insoluble in water. Miscible with alcohol and with ether. Boils at about 180

.
Assay—
When examined by gas–liquid chromatography, with the use of suitable apparatus and conditions, it shows a purity of not less than 98%.
Density:
between 1.299 and 1.301.
Residue on evaporation—
Evaporate 80 mL on a steam bath, and dry at 105

for 1 hour: the residue weighs not more than 50 mg (0.005%).
Acidity—
Add
phenolphthalein TS to 25 mL of methanol, and titrate with 0.02 N alcoholic potassium hydroxide VS until a faint pink color persists for 15 seconds. Pipet 25 mL of test specimen into the solution; mix, avoiding exposure to the atmosphere; and titrate with 0.02 N alcoholic potassium hydroxide VS: not more than 2.2 mL is required to restore the pink color (about 0.005%).
2,8-Dichlorodibenzo-p-dioxin,

C
12H
6Cl
2O
2—
253.08


[
67478-04-0]—Use a suitable grade.
[NOTE—A suitable grade is available as a mixture of the 2,7- and 2,8-isomers, catalog number ED-926, from Cambridge Isotope Laboratories, Inc.,
www.isotope.com.
]
2,8-Dichlorodibenzofuran,

C
12H
5Cl
2O—
236.07—Off-white fibers.
Assay—
MOBILE PHASE—
Prepare a mixture of acetonitrile and water (80: 20).
PROCEDURE—
Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography  621
621
) equipped with a 254-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. The area of the C
12H
5Cl
2O peak is not less than 99.9% of the total peak area.
1,2-Dichloroethane
—See Ethylene Dichloride.
Dichlorofluorescein,

C
20H
10Cl
2O
5—
401.20—
[NOTE—This specification covers both the 4,5- and 2,7-isomers of dichlorofluorescein, either of which is suitable for the preparation of
dichlorofluorescein TS.
] A weak orange-colored, crystalline powder. Sparingly soluble in water; soluble in alcohol and in solutions of alkali hydroxides.
Residue on ignition (Reagent test)—
Ignite 200 mg with 5 drops of sulfuric acid: the residue weighs not more than 1 mg (0.5%).
Sensitiveness—
Dissolve 100 mg in 60 mL of alcohol, add 2.5 mL of 0.1 N sodium hydroxide, and dilute with water to 100 mL. Add 1 mL of this solution to a solution of potassium iodide prepared by dissolving 100 mg of potassium iodide, previously dried at 105

to constant weight and accurately weighed, in 50 mL of water containing 1 mL of glacial acetic acid, and titrate with 0.1 N silver nitrate VS until the color of the precipitate changes from pale yellowish orange to pink. The volume of 0.1 N silver nitrate consumed is not more than 0.10 mL greater than the calculated volume, the calculated volume being based upon the KI content of the dried specimen as determined in the
Assay under
Potassium Iodide (USP monograph).
Dichlorofluoromethane,

CHCl
2F—
102.92—Colorless gas.
Assay—
Inject an appropriate specimen into a gas chromatograph (see
Chromatography  621
621
) equipped with a thermal-conductivity detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.53-mm × 30-m capillary column coated with a 5-µm layer of phase G2; the injection port temperature is maintained at 200

; the detector temperature is maintained at 200

; and the column temperature is maintained at 0

and programmed to rise 5

per minute to 40

, and then to rise at 10

per minute to 180

. The area of the CHCl
2F peak is not less than 98% of the total peak area.
Dichloromethane
—Use Methylene Chloride.
2,4-Dichloro-1-naphthol,

C
10H
6OCl
2—
213.06—Light tan powder.
Melting range  741
741 :
:

between 103

and 107

, but the range between beginning and end of melting does not exceed 2

.
2,4-Dichlorophenol,

C
6H
4Cl
2O—
163.00—White to off-white crystalline solid.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 230

; the detector temperature is maintained at 300

; the column temperature is maintained at 130

and programmed to rise 10

per minute to 280

. The area of the C
6H
4Cl
2O peak is not less than 98.5% of the total peak area.
2,6-Dichlorophenol-indophenol Sodium
(
2,
6-Dichloro-indophenol Sodium),

O:C
6H
2Cl
2:NC
6H
4ONa with about 2H
2O—
290.08 (anhydrous)—Use ACS reagent grade.
2,6-Dichlorophenylacetic Acid,

C
8H
6Cl
2O
2—
205.04—White powder.
Assay—
Inject an appropriate specimen into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 250

; the detector temperature is maintained at 300

; and the column temperature is maintained at 150

and programmed to rise 10

per minute to 280

. The area of the C
8H
6Cl
2O
2 peak is not less than 97% of the total peak area.
2,6-Dichloroquinone-chlorimide
(
2,
6-Dichloro-N-chloro-p-benzoquinone Imine),

O:C
6H
2Cl
2:NCl—
210.44—Pale yellow, crystalline powder. Insoluble in water; soluble in alcohol and in dilute alkali hydroxide solutions.
Solubility in alcohol—
A solution of 100 mg in 10 mL of alcohol is complete and clear.
Residue on ignition—
Ignite 500 mg with 0.5 mL of sulfuric acid: the residue weighs not more than 1 mg (0.2%).
Sensitiveness—
It meets the requirements of the test for Sensitiveness under 2,6-Dibromoquinone-chlorimide.
Dicyclohexyl,

C
12H
22—
166.31—Use a suitable grade.
Change to read:
Dicyclohexylamine,

(C
6H
11)
2NH—
181.32—Clear, strongly alkaline liquid.

 USP29
USP29 Sparingly soluble in water. Miscible with common organic solvents. Density: 0.9104. Solidifies at
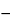
0.1

; melts at about 20

.
Assay—
Accurately weigh about 400 mg in a tared, small weighing bottle equipped with a well-fitting closure. Transfer the stoppered bottle to a 250-mL beaker, add sufficient glacial acetic acid TS to cover the bottle, and open the bottle under the surface of the acid. Add
crystal violet TS, and titrate with 0.1 N perchloric acid VS. Each mL of 0.1 N perchloric acid is equivalent to 18.13 mg of (C
6H
11)
2NH. Not less than 98% is found.
Boiling range (Reagent test):

between 255

and 257

.
Dicyclohexyl Phthalate,

C
20H
26O
4—
330.42


[
84-61-7].
Diethylamine,

(C
2H
5)
2NH—
73.14—Colorless, flammable, strongly alkaline liquid. Miscible with water and with alcohol. Forms a hydrate with water.
May be irritating to skin and mucous membranes. Store in well-closed containers. Use ACS reagent grade.
N,N-Diethylaniline,

C
6H
5N(C
2H
5)
2—
149.23—Light yellow to amber liquid.
Assay—
Inject an appropriate specimen (about 0.2 µL) into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas flowing at about 40 mL per minute. The following conditions have been found suitable: a 3-mm × 1.8-m stainless steel column containing 20% phase G16 on support S1A; the injection port temperature is maintained at 250

; the column temperature is maintained at 140

and programmed to rise 6

per minute to 200

. The detector temperature is maintained at 310

. The area of the
N,
N-diethylaniline peak having a retention time of about 4.9 minutes is not less than 99% of the total peak area.
Diethylene Glycol,

C
4H
10O
3—
106.12


[
11-46-6]—A colorless to faintly yellow, viscous, hygroscopic liquid. Miscible with water, with alcohol, with ether, and with acetone. Insoluble in benzene and in carbon tetrachloride.
Acidity—
Transfer 54 mL (60 g) to a 250-mL conical flask, add
phenolphthalein TS, and titrate with 0.02 N alcoholic potassium hydroxide VS to the production of a pink color that is stable for at least 15 seconds: not more than 2.5 mL is consumed (0.005% as CH
3COOH).
Residue on ignition  281
281 —
—
Transfer 50 g to a tared platinum dish, heat the dish gently until the vapors ignite, and allow the specimen to burn completely. Ignite the residue at 800 ± 25

, cool, and weigh: the residue weighs not more than 2.5 mg (0.005%).
Diethylene Glycol Succinate Polyester,

(OCH
2CH
2OCH
2CH
2OOCCH
2CH
2COO)
n—Clear, viscous liquid. Soluble in chloroform. Is stabilized by modification of the polyester, diethylene glycol succinate, to render it suitable for use in gas-liquid chromatography to a temperature of 200

.
Diethylenetriamine,

C
4H
13N
3—
103.17—Colorless liquid.
Assay—
Inject an appropriate specimen into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with G2. The injection port temperature is maintained at 200

; the column temperature is maintained at 100

and programmed to rise 10

per minute to 250

and held there for 5 minutes. The detector temperature is maintained at 300

. The area of the main peak is not less than 95% of the total peak area.
Di(2-ethylhexyl)phthalate
[
Bis(
2-ethylhexyl)
phthalate],

C
24H
38O
4—
390.56—Use a suitable grade.
Diethylpyrocarbonate,

C
6H
10O
5—
162.14


[
1609-47-8]—Clear, colorless liquid. Use a suitable grade.
Digitonin,

C
56H
92O
29—
1229.31—White, crystalline powder. Almost insoluble in water; soluble in warm alcohol, and in glacial and in 75 percent acetic acid; insoluble in chloroform and in ether. Melts at about 230

, with decomposition.
Specific rotation  781
781 :
:

between
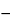
47

and
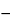
49

, determined in a solution in 75 percent acetic acid containing 100 mg per mL.
Solubility in alcohol—
A solution of 500 mg in 20 mL of warm alcohol is colorless and complete.
Loss on drying  731
731 —
—
Dry it at 105

to constant weight: it loses not more than 6% of its weight.
Residue on ignition (Reagent test):

not more than 0.3%.
Digoxigenin Bisdigitoxoside
—Use a suitable grade.
[NOTE—A suitable grade is available from Crescent Chemical Co., Inc., 1324 Motors Parkway, Hauppauge, NY 11788.]
10-11-Dihydrocarbamazepine,

C
15H
14N
2O—
238.28—White crystals.
Assay—
When tested by thin-layer chromatography, with the use of plates coated with chromatographic silica gel mixture, a developing system consisting of toluene and methanol (80:20), and examined visually and under long-wavelength UV light, a single spot is exhibited.
Dihydroquinidine Hydrochloride

C
20H
27ClN
2O
2—
362.89—Rhombic plates. Freely soluble in methanol and in chloroform.
Assay—
MOBILE PHASE—
Prepare a mixture of water, acetonitrile, diethylamine, and methanesulfonic acid (860:100:20:20).
PROCEDURE—
Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography  621
621
) equipped with a 235-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. The area of the C
20H
27ClN
2O
2 peak is not less than 97.5% of the total peak area.
Dihydroquinine
(
Hydroquinine),

C
20H
26N
2O
2—
326.43—Freely soluble in acetone, in alcohol, and in chloroform; almost insoluble in water.
Assay—
MOBILE PHASE—
Prepare a mixture of water, acetonitrile, diethylamine, and methanesulfonic acid (860:100:20:20) and methanol (82:18).
PROCEDURE—
Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography  621
621
) equipped with a 235-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 1.5 mL per minute. The area of the C
20H
26N
2O
2 peak is not less than 97.5% of the total peak area.
2,5-Dihydroxybenzoic Acid,

C
7H
6O
4—
154.12—Off-white powder. Freely soluble in alcohol yielding a clear, very pale yellow solution.
Assay—
Dissolve about 75 mg, accurately weighed, in 30 mL of methanol. Slowly add 40 mL of water. Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically. Perform a blank determination and make any necessary correction. Each mL of 0.1 N sodium hydroxide is equivalent to 15.41 mg of C7H6O4. Not less than 99% is found.
Change to read:
Diiodofluorescein,

C
20H
10I
2O
5—
584.10—Orange-red

 USP29
USP29 powder. Slightly soluble in water; soluble in alcohol and in solutions of alkali hydroxides.
Residue on ignition—
Ignite 200 mg with 5 drops of sulfuric acid: the weight of the residue does not exceed 1.0 mg (0.5%).
Sensitiveness—
Accurately weigh about 100 mg of potassium iodide, previously dried at 105

to constant weight, and dissolve it in 50 mL of water. Add 1 mL of diiodofluorescein TS prepared from the test specimen and 1 mL of glacial acetic acid, and titrate with 0.1 N silver nitrate VS until the color of the precipitate changes from brownish red to a bluish red. The volume of 0.1 N silver nitrate consumed is not in excess of 0.10 mL over the calculated volume, based on the KI content of the dried potassium iodide determined as follows. Dissolve about 500 mg of potassium iodide, accurately weighed, in about 10 mL of water, and add 35 mL of hydrochloric acid and 5 mL of chloroform. Titrate with 0.05 M potassium iodate VS until the purple color of iodine disappears from the chloroform. Add the last portions of the iodate solution dropwise, agitating vigorously and continuously. After the chloroform has been decolorized, allow the mixture to stand for 5 minutes. If the chloroform develops a purple color, titrate further with the iodate solution. Each mL of 0.05 M potassium iodate is equivalent to 16.60 mg of KI.
Diisodecyl Phthalate
[
Bis(
isodecyl)
phthalate],

C
28H
46O
4—
446.66—Use a suitable grade.
Diisopropyl Ether
(
Isopropyl Ether)

[(CH
3)
2CH]
2O—
102.17—Colorless, mobile liquid. Slightly soluble in water. Miscible with alcohol and with ether. [
Caution—It is highly flammable. Do not use where it may be ignited. Do not evaporate to the point of near dryness,
since it tends to form explosive peroxides.]
Specific gravity:

between 0.716 and 0.720.
Peroxides—
To 10 mL, contained in a clean, glass-stoppered cylinder previously rinsed with a portion of the ether under examination, add 1 mL of freshly prepared potassium iodide solution (1 in 10). Shake, and allow to stand for 1 minute: no yellow color is observed in either layer (about 0.001% as H2O2).
Residue on evaporation—
[
Note—If peroxide is present,
do not carry out this procedure.] Evaporate 14 mL (10 g) from a tared shallow dish, and dry at 105

for 1 hour: the residue weighs not more than 1 mg (0.01%).
Acidity—
Add 2 drops of
bromothymol blue TS to 10 mL of water in a glass-stoppered, 50-mL flask, and titrate with 0.010 N sodium hydroxide until a blue color persists after vigorous shaking. Add 5 mL of Diisopropyl Ether, and titrate with 0.010 N sodium hydroxide: not more than 0.30 mL is required to restore the blue color (0.005% as CH
3COOH).
NOTE—For spectrophotometric determinations, use diisopropyl ether that meets the following additional requirement:
Absorbance—
Its absorbance at 255 nm, in a 10-mm quartz cell, does not exceed 0.2, water being used as the blank.
Diisopropylamine,

[(CH
3)
2CH]
2NH—
101.19—Colorless liquid.
Assay—
Not less than 98% of C
6H
15N is found, a suitable gas chromatograph equipped with a flame-ionization detector being used. The following conditions have been found suitable: a 3.2-mm × 1.83-m stainless steel column is packed with a cross-linked polystyrene support; the injection port temperature is maintained at 250

and the detector at 310

; the column temperature is programmed to rise at 10

per minute from 50

to 220

.
Diisopropylethylamine
(
N,
N-Diisopropylethylamine),

C
8H
19N—
129.24—Clear, colorless liquid. Soluble in glacial acetic acid.
Assay—
Accurately weigh about 500 mg, dissolve in 50 mL of glacial acetic acid, mix, add
crystal violet TS, and titrate with 0.1 N perchloric acid VS. Each mL of 0.1 N perchloric acid is equivalent to 12.92 mg of C
8H
19N. Not less than 98% is found.
1,2-Dilinoleoyl-3-oleoyl-rac-glycerol,

C
57H
100O
6—
881.4 


[
2190-21-8]—Use a suitable grade.
1,2-Dilinoleoyl-3-palmitoyl-rac-glycerol,

C
55H
98O
6—
855.4 


[
2190-15-0]—Use a suitable grade.
2,5-Dimethoxybenzaldehyde,

C
9H
10O
3—
166.17—Off-white crystals.
Assay—
Inject an appropriate specimen into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, nitrogen being used as the carrier gas. The following conditions have been found suitable: a 0.3-mm × 30-m capillary column coated with phase G1; the injection port temperature is maintained at 270

; the detector temperature is maintained at 300

; the column temperature is maintained at 150

and programmed to rise 10

per minute to 270

. The area of the main peak is not less than 97% of the total peak area.
Change to read:
1,2-Dimethoxyethane,

C
4H
10O
2—
90.12—Clear, colorless liquid.

 USP29
USP29 Miscible with water and with alcohol. Soluble in hydrocarbon solvents.
May form peroxides on standing.
Boiling range (Reagent test)—
Not less than 95% distills between 83

and 86

.
Acidity—
To 20 mL add
bromophenol blue TS, and titrate with 0.020 N sodium hydroxide: not more than 2.0 mL is consumed (about 0.015% as CH
3COOH).
Dimethoxymethane
(
Formaldehyde Dimethyl Acetal,
Methylal),

C
3H
8O
2—
76.10


[
109-87-5]—Use a suitable grade.
(3,4-Dimethoxyphenyl)-acetonitrile
(
Homoveratronitrile),

C
10H
11NO
2—
177.20—Off-white fibers.
Dimethyl Phthalate,

C
10H
10O
4—
194.19—Viscous, colorless liquid.
Assay—
M
OBILE PHASE—Prepare a filtered and degassed mixture of chromatographic
n-heptane and
n-propyl alcohol (HPLC grade) (97:3). Make adjustments if necessary (see
System Suitability under
Chromatography  621
621
).
STANDARD SOLUTION—Dissolve a suitable quantity of dimethyl phthalate in Mobile phase to obtain a solution having a known concentration of about 0.26 mg per mL.
P
ROCEDURE—Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography  621
621
) equipped with a 238-nm detector and a 4.6-mm × 25-cm column that contains packing L10. The flow rate is about 1.0 mL per minute. The area of the dimethyl phthalate peak is not less than 99% of the total peak area.
Dimethyl Sulfone
(
Methyl Sulfone),

(CH
3)
2SO
2—
94.13—White crystals.
Dimethyl Sulfoxide
(Methyl Sulfoxide),

(CH
3)
2SO—
78.13—Use ACS reagent grade methyl sulfoxide.
Dimethyl Sulfoxide, Spectrophotometric Grade—
Use methyl sulfoxide ACS spectrophotometric reagent grade.
N,N-Dimethylacetamide,

C
4H
9NO—
87.12—Clear, colorless liquid. Miscible with water and with many organic solvents.
Assay—
When examined by gas-liquid chromatography, with the use of suitable apparatus and conditions, it shows a purity of not less than 99%.
Residue on evaporation—
Evaporate 215 mL on a steam bath, and dry at 105

for 1 hour: the residue weighs not more than 2 mg (0.001%).
pH of 20% solution—
Weigh 20 g of it into a 100-mL volumetric flask, and dilute with carbon dioxide-free water to volume: the solution shows a pH between 4.0 and 7.0.
Ultraviolet absorbance—
Determine its absorbance throughout the range 270 to 400 nm, using a 1-cm cell, a suitable spectrophotometer, and water to set the instrument: the absorbance does not exceed 1.00 at 270 nm, 0.30 at 280 nm, 0.15 at 290 nm, 0.05 at 310 nm, 0.03 at 320 nm, and 0.01 at 360 to 400 nm.
p-Dimethylaminoazobenzene
(
Methyl Yellow,
Butter Yellow),

C
6H
5N:NC
6H
4N(CH
3)
2—
225.29—Yellow leaflets or yellow, crystalline powder.
Solubility—
Insoluble in water; sparingly soluble in chloroform, in ether, or in fatty oils. Dissolve 100 mg in 20 mL of alcohol: the solution is complete or practically so and clear.
Sensitiveness—
Add 0.05 mL of an alcohol solution (1 in 200) and 2 g of ammonium chloride to 25 mL of carbon dioxide-free water: the lemon-yellow color of the solution is changed to orange by the addition of 0.05 mL of 0.1 N hydrochloric acid and restored on the subsequent addition of 0.05 mL of 0.1 N sodium hydroxide.
p-Dimethylaminobenzaldehyde,

(CH
3)
2NC
6H
4CHO—
149.19— Use ACS reagent grade.
p-Dimethylaminocinnamaldehyde,

(CH
3)
2NC
6H
4CH:CHCHO—
175.23—Orange-yellow powder. Soluble in acetone, in alcohol, and in benzene.
Dimethylaminophenol
(
meta isomer),

C
8H
11NO—
137.18—Purplish–black, gray, or tan-colored, crystalline solid.
2,6-Dimethylaniline,

C
8H
11N—
121.18—Yellow liquid.
N,N-Dimethylaniline,

C
6H
5N(CH
3)
2—
121.18—Light yellow liquid. Clear, colorless liquid when freshly distilled, but acquiring a reddish to reddish–brown color. Specific gravity: about 0.960. Freezing point about 2

. Insoluble in water; soluble in alcohol, in chloroform, in ether, and in dilute mineral acids.
Assay—
Inject an appropriate specimen (about 0.2 µL) into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas flowing at about 40 mL per minute. The following conditions have been found suitable: a 3-mm × 1.8-m stainless steel column containing 20% phase G16 on support S1A; the injection port temperature is maintained at 250

; the column temperature is maintained at 50

and programmed to rise 10

per minute to 200

. The detector temperature is maintained at 310

. The area of the
N,
N-dimethylaniline peak having a retention time of about 11.5 minutes is not less than 99% of the total peak area.
Boiling range (Reagent test)—
Distill 100 mL: the difference between the temperatures observed, when 1 mL and 95 mL have distilled, is not more than 2.5

. Its boiling temperature at a pressure of 760 mm of mercury is 194.2

.
Hydrocarbons—
Dissolve 5 mL in a mixture of 10 mL of hydrochloric acid and 15 mL of water: a clear solution results and it remains clear on cooling to about 10

.
Aniline or monomethylaniline—
Place 5 mL in a glass-stoppered flask, add 5 mL of a solution of acetic anhydride in benzene (1 in 10), mix, and allow to stand for 30 minutes. Add 30.0 mL of 0.5 N sodium hydroxide VS, shake the mixture, add
phenolphthalein TS, and titrate with 0.5 N hydrochloric acid VS. Perform a blank determination, and make any necessary correction. Not more than 0.30 mL of 0.5 N sodium hydroxide is consumed by the test specimen.
3,4-Dimethylbenzophenone,

C
15H
14O—
210.27—White chunks melting at about 45

.
Assay—
Inject an appropriate specimen into a suitable gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with phase G1: the detector temperature and the injection port temperature are maintained at 300

; the column temperature is maintained at 180

and programmed to rise at the rate of 10

per minute to 280

and held at that temperature for 10 minutes. The area of the main peak is not less than 99% of the total peak area.
5,5-Dimethyl-1,3-cyclohexanedione,

C
8H
12O
2—
140.18—White, crystalline solid. Slightly soluble in water; soluble in alcohol, in methanol, in chloroform, and in acetic acid.
1,5-Dimethyl-1,5-diazaundecamethylene polymethobromide (Polybrene),



[
28728-55-4]—This is a positively charged polymer. It is available as an off-white, crystalline solid or powder and is extremely hygroscopic. Use a suitable reagent grade.
[NOTE—Commercially available as Polybrene.]
Dimethylethyl(3-hydroxyphenyl)ammonium Chloride
—See Edrophonium Chloride.
Dimethylformamide
(
N,
N-Dimethylformamide),

HCON(CH
3)
2—
73.09—Use ACS reagent grade.
N,N-Dimethylformamide Diethyl Acetal
—
147.22 


[
1188-33-6]—Use a suitable grade.
1,3-Dimethyl-2-imidazolidinone,

C
5H
10N
2O—
114.15


[
80-73-9]—Use a suitable grade.
1,9-Dimethyl-Methylene Blue,

C
36H
46Cl
4N
6OS
2Zn—
850.1


[
23481-50-7]—Dark green powder. Use a suitable grade.
N,N-Dimethyl-1-naphthylamine,

C
12H
13N—
171.24—Pale yellow to yellow, aromatic liquid. Soluble in alcohol and in ether.
Assay—
Transfer about 250 mg, accurately weighed, to a suitable beaker, add 100 mL of glacial acetic acid, and dissolve by stirring. When solution is complete, titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 17.12 mg of C12H13N. Not less than 98% is found.
Sulfanilamide test—
Dissolve 20 mg of
USP Sulfanilamide RS in 100 mL of water to obtain the
Sulfanilamide solution. Into two 150-mL beakers pipet 1.0 mL and 2.5 mL of the
Sulfanilamide solution, respectively. Dilute with water to 90 mL. To provide a blank, place 90 mL of water in a third beaker. To each beaker add 8.0 mL of trichloroacetic acid solution (3 in 20) and 1.0 mL of sodium nitrite solution (1 in 1000). Stir the solutions for 5 minutes, then add 10 mL of
acetate buffer TS, and 1.0 mL of a 1 in 1000 solution of
N,
N-dimethyl-1-naphthylamine in alcohol. The pH is about 5 to 6, using pH paper. Stir for an additional 5 minutes, then add 20 mL of glacial acetic acid. The pH is about 3 to 4, using pH paper. In comparison with the blank, the beaker containing 1.0 mL of the
Sulfanilamide solution shows a pink color, while the other beaker shows a deep pink to red color.
N,N-Dimethyloctylamine,

C
10H
23N—
157.30—Colorless liquid.
2,5-Dimethylphenol,

C
8H
10O—
122.16


[
95-87-4]—Use a suitable grade.
2,6-Dimethylphenol,

(CH
3)
2C
6H
3OH—
122.16—White to pale yellow crystalline solid.
Assay—
Inject a 1 in 3 solution of it in xylene into a suitable gas chromatograph equipped with a flame-ionization detector, helium being used as the carrier gas at a flow rate of about 40 mL per minute. The following conditions have been found suitable: a 3.2-mm × 1.83-m stainless steel column packed with 10% phase G25 on support S1A; the injection port is maintained at about 250

and the detector at about 310

; the column temperature is programmed to rise at 8

per minute from 100

to 200

. Similarly inject a specimen of xylene. The area of the C
8H
10O peak is not less than 98% of the total peak area corrected for xylene.
3,5-Dimethylphenol,

C
8H
10O—
122.16


[
108-68-9]—Use a suitable grade.
N,N-Dimethyl-p-phenylenediamine Dihydrochloride,

(CH
3)
2NC
6H
4NH
2·2HCl—
209.12—Nearly white, fine, crystalline, hygroscopic solid that may have a pinkish cast. Freely soluble in water; soluble in alcohol.
Assay—
Transfer about 400 mg, accurately weighed, to a 250-mL beaker, and dissolve in about 75 mL of water. Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically. Each mL of 0.1 N sodium hydroxide is equivalent to 10.46 mg of C8H12N2·2HCl. Not less than 98% is found.
Solubility—
A solution of 1 g in 10 mL of water produces not more than a slight haze.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl Tetrazolium Bromide,

C
18H
16N
5SBr—
414.3 


[
298-93-1]—Yellow to orange powder. Use a suitable grade.
m-Dinitrobenzene,

C
6H
4(NO
2)
2—
168.11—Pale yellow crystals or crystalline powder. Almost insoluble in cold water; slightly soluble in hot water. Soluble in chloroform and in benzene; sparingly soluble in alcohol. Is volatile in steam.
Residue on ignition (Reagent test):

not more than 0.5%.
3,5-Dinitrobenzoyl Chloride,

C
7H
3ClN
2O
5—
230.56—Pale yellow, crystalline powder. Freely soluble in dilute sodium hydroxide solutions; soluble in alcohol.
[Caution—Corrosive, moisture-sensitive, lachrymator, and possible mutagen. Store under nitrogen.
]
Solubility in sodium hydroxide—
A solution of 500 mg in 25 mL of 1 N sodium hydroxide is clear or not more than faintly turbid.
Residue on ignition—
Ignite 1 g with 0.5 mL of sulfuric acid: the residue weighs not more than 1 mg (0.1%).
2,4-Dinitrochlorobenzene,

C
6H
3(NO
2)
2Cl—
202.55—Yellow to brownish-yellow crystals. Insoluble in water; soluble in hot alcohol, in ether, and in benzene.
Residue on ignition—
Ignite 500 mg with 5 drops of sulfuric acid: the residue weighs not more than 1 mg (0.2%).
2,4-Dinitrofluorobenzene
(1-Fluoro-2,4-dinitrobenzene),

C
6H
3FN
2O
4—
186.10—Light yellow solid. Use a suitable grade.
2,4-Dinitrophenylhydrazine,

2,4-C
6H
3(NO
2)
2NHNH
2—
198.14—Orange-red crystals, which under the microscope appear individually to be lemon-yellow, lath-like needles. Very slightly soluble in water; slightly soluble in alcohol; moderately soluble in dilute inorganic acids.
Solubility in sulfuric acid—
Dissolve 500 mg in a mixture of 25 mL of sulfuric acid and 25 mL of water: the solution is clear or not more than slightly turbid.
Residue on ignition (Reagent test):

negligible, from 500 mg.
Dioctyl Sodium Sulfosuccinate
—Use Docusate Sodium.
Dioxane
(
Diethylene Dioxide; 1,
4-Dioxane),

C
4H
8O
2—
88.11—Use ACS reagent grade.
Diphenyl Ether
(
Phenyl Ether),

(C
6H
5)
2O—
170.21—A colorless liquid. Insoluble in water; soluble in glacial acetic acid and in most organic solvents. Boils at about 259

.
Diphenylamine,

(C
6H
5)
2NH—
169.22—Use ACS reagent grade.
Diphenylborinic Acid, Ethanolamine Ester,
(
2-Aminoethyl Diphenylborinate)

C
14H
16BNO—
225.09—White, crystalline powder. Use a suitable grade.
Diphenylcarbazide,

(C
6H
5NHNH)
2CO—
242.28—Use ACS reagent grade 1,5-Diphenylcarbohydrazide.
Diphenylcarbazone
[
Diphenylcarbazone compd. with s-Diphenylcarbazide(
1:1)],

C
6H
5NHNHCON:NC
6H
5·C
6H
5NHNHCONHNHC
6H
5—
482.54—Use ACS reagent grade Diphenylcarbazone Compound with
s-Diphenylcarbazide (1:1).
2,2-Diphenylglycine,

C
14H
13NO
2—
227.26—Off-white powder. Melts at about 244

, with decomposition.
Assay—
Dissolve about 115 mg, accurately weighed, in 30 mL of methanol. Slowly add about 20 mL of water, heating slightly if necessary for complete solution. Titrate with 0.1 N sodium hydroxide VS, determining the endpoint potentiometrically. Perform a blank determination and make any necessary correction. Each mL of 0.1 N sodium hydroxide is equivalent to 22.73 mg of C14H13NO2. Not less than 98.0% is found.
Dipropyl Phthalate,

C
14H
18O
4—
250.29—Viscous, colorless liquid.
Assay—
MOBILE PHASE—
Prepare a mixture of acetonitrile and water (52:48).
PROCEDURE—
Inject about 20 µL into a suitable liquid chromatograph (see
Chromatography  621
621
) equipped with a 230-nm detector and a 4.6-mm × 15-cm column that contains packing L1. The flow rate is about 2.0 mL per minute. The area of the C
14H
18O
4 peak is not less than 99% of the total peak area.
4,4¢-Dipyridyl Dihydrochloride,

C
10H
8N
2·2HCl—
229.11—White to off-white crystals.
Assay—
Inject an appropriate volume into a gas chromatograph (see
Chromatography  621
621
) equipped with a flame-ionization detector, helium being used as the carrier gas. The following conditions have been found suitable: a 0.25-mm × 30-m capillary column coated with a 1-µm layer of phase G2; the injection port temperature is maintained at 230

; the detector temperature is maintained at 300

; and the column temperature is maintained at 160

and programmed to rise 10

per minute to 260

. The area of the C
10H
8N
2·HCl peak is not less than 98.5% of the total peak area.
Disodium Chromotropate
(4,5-Dihydroxy-2,7-naphthalenedisulfonic Acid, Disodium Salt),

C
10H
6O
8S
2Na
2·2H
2O—
400.29—Use ACS reagent grade Chromotropic Acid Disodium Salt.
Disodium Phosphate
—See Sodium Phosphate.
5,5¢-Dithiobis (2-nitrobenzoic Acid)
(
3-Carboxy-4-nitrophenyl disulfide; Ellman's reagent),

C
14H
8N
2O
8S
2—
396.35—Yellow powder, melting at about 242

. Sparingly soluble in alcohol.
Dithiothreitol
(
Cleland's Reagent, threo-1,4-dimercapto-2,3-butanediol, DTT),

HSCH
2CH(OH)CH(OH)CH
2SH—
154.25—Slightly hygroscopic needles from ether. Freely soluble in water, in alcohol, in acetone, in ethyl acetate, and in ether.
Dithizone
(
Diphenylthiocarbazone; Phenylazothioformic Acid 2-Phenylhydrazide),

C
6H
5N:NCSNHNHC
6H
5—
256.33—Use ACS reagent grade.
1-Dodecanol
(Dodecyl Alcohol),

CH
3(CH
2)
11OH—
186.33—A clear, colorless liquid. Crystallizes as leaflets from dilute alcohol solution. Use ACS reagent grade.
3-(Dodecyldimethylammonio)propanesulfonate (Lauryl sulfobetaine, N,N-dimethyl-N-dodecyl-N-(3-sulfopropyl)ammonium betaine),

C
17H
37NO
3S—
335.54 


[
14933-08-5]—Use a suitable grade.
Dodecyl Lithium Sulfate (Lithium Dodecyl Sulfate, Lithium Lauryl Sulfate),

C
12H
25LiO
4S—
272.3


[
2044-56-6]—White to off-white powder, clear to slightly hazy, colorless to faint yellow solution in water at 50 mg per mL at ambient temperature. The UV absorbance of a 0.1 M solution is less than 0.05 at both 260 and 280 nm. The pH of a 0.1 M solution in water is 7.0 ± 0.5. Use a suitable grade.
Dodecyl Sodium Sulfonate
(Sodium 1-Dodecanesulfonate; 1-Dodecanesulfonic Acid, Sodium Salt),

C
12H
25SO
3Na—
272.38


[
2386-53-0]—White, powdery solid. One g dissolves in 50 mL of warm water to yield a clear, colorless solution. Use a suitable grade.
Dodecyltriethylammonium Phosphate, 0.5 M,

[C
12H
25N·(C
2H
5)
3]
3PO
4—
906.52—Use a suitable grade.
[NOTE—A suitable grade is available as Ion Pair Cocktail Q12 (catalogue number 404031) from Regis Technologies, Inc.,
www.registech.com.
]
Drabkin's Reagent—
The reagent consists of 100 parts of sodium bicarbonate, 20 parts of potassium ferricyanide, and 5 parts of potassium cyanide.
[Caution—The reagent is HIGHLY TOXIC. Very toxic by inhalation, in contact with skin, and if swallowed, and there is a risk of serious damage to eyes. Wear suitable protective clothing, gloves, and eye and face protection. Do not mix with acids. Contact with acids liberates a very toxic gas. If ingested, perform gastric lavage, and call a physician.
]
[NOTE—The reagent can be obtained from many manufacturers and suppliers. Some examples of manufacturers or suppliers are the following: Sigma Chemical Co., St. Louis, MO; and CIMA Scientific, Dallas, TX.]