(C
17H
23NO
3)
2·H
2SO
4·H
2O





694.83
Benzeneacetic acid,
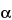
-(hydroxymethyl)-, 8-methyl-8-azabicyclo[3.2.1]oct-3-yl ester,
endo-(±)-, sulfate (2:1) (salt), monohydrate.
1
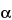 H
H,5
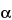 H
H-Tropan-3-
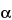
-ol (±)-tropate (ester), sulfate (2:1) (salt) monohydrate



[
5908-99-6].
Anhydrous
676.83



[
55-48-1].
Packaging and storage—
Preserve in tight containers.
Identification—
B:
A solution (1 in 20) meets the requirements of the tests for
Sulfate  191
191
.
Melting temperature, Class Ia  741
741 :
:
not lower than 187

, determined after drying at 120

for 4 hours.
[NOTE—Since anhydrous Atropine Sulfate is hygroscopic, determine its melting temperature promptly on a specimen placed in the capillary tube immediately after drying.
]
Angular rotation  781A
781A —
—
The observed rotation, in degrees, multiplied by 200, and divided by the length, in mm, of the polarimeter tube used, is between
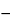
0.60

and +0.05

(limit of hyoscyamine).
Test solution—
Dissolve 1 g, accurately weighed, in water to make a volume of 20 mL at 25

.
Acidity—
Dissolve 1.0 g in 20 mL of water, add 1 drop of
methyl red TS, and titrate with 0.020 N sodium hydroxide: not more than 0.30 mL is required to produce a yellow color.
Organic volatile impurities, Method I  467
467 :
:
meets the requirements.
Other alkaloids—
Dissolve 150 mg in 10 mL of water. To 5 mL of the solution add a few drops of
platinic chloride TS: no precipitate is formed. To the remaining 5 mL of the solution add 2 mL of 6 N ammonium hydroxide, and shake vigorously: a slight opalescence may develop but no turbidity is produced.
Assay—
Dissolve about 1 g of Atropine Sulfate, accurately weighed, in 50 mL of glacial acetic acid, and titrate with 0.1 N perchloric acid VS, determining the endpoint potentiometrically. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 67.68 mg of (C17H23 NO3)2·H2SO4.
Auxiliary Information—
Staff Liaison :
Ravi Ravichandran, Ph.D., Senior Scientist
Expert Committee : (MDPP05) Monograph Development-Psychiatrics and Psychoactives
USP29–NF24 Page 219
Pharmacopeial Forum : Volume No. 29(6) Page 1847
Phone Number : 1-301-816-8330