B:
Transfer 0.25 g of Powdered Cellulose, accurately weighed to 0.1 mg, to a 125-mL conical flask. Add 25.0 mL of water and 25.0 mL of 1.0 M cupriethylenediamine hydroxide solution. Immediately purge the solution with nitrogen, insert the stopper, and shake on a wrist action shaker or other suitable mechanical shaker until completely dissolved. Transfer 7.0 mL of the solution to a calibrated number 150 Cannon-Fenske, or equivalent, viscosimeter. Allow the solution to equilibrate at 25 ± 0.1

for not less than 5 minutes. Time the flow between the two marks on the viscosimeter, and record the flow time,
t1, in seconds. Calculate the kinematic viscosity, (
KV)
1, of the Powdered Cellulose taken by the formula:
t1(k1),
in which
k1 is the viscosimeter constant (see
Viscosity  911
911
). Obtain the flow time,
t2, for a 0.5 M cupriethylenediamine hydroxide solution using a number 100 Cannon-Fenske, or equivalent, viscosimeter. Calculate the kinematic viscosity, (
KV)
2, of the solvent by the formula:
t2(k2),
in which
k2 is the viscosimeter constant. Determine the relative viscosity,
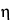 rel
rel, of the Powdered Cellulose specimen taken by the formula:
(KV)1 / (KV)2.
Determine the intrinsic viscosity, [
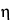
]
c, by interpolation, using the
Intrinsic Viscosity Table in the
Reference Tables section. Calculate the degree of polymerization,
P, by the formula:
(95)[
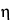
]
c /
WS[(l00 – %
LOD)/100],
in which
WS is the weight, in g, of the Powdered Cellulose taken; and %
LOD is the value obtained from the test for
Loss on drying. The degree of polymerization is greater than 440.