Packaging and storage—
Preserve in tight containers.
Identification—
A:
To a solution of about 5 mg in 5 mL of water add 5 mL of ammonium reineckate solution (1 in 30), and shake vigorously for 1 minute: a red precipitate is formed, and it is soluble in acetone.
B:
To 500 mg add 10 mL of
alcoholic potassium hydroxide TS, and boil gently for 1 to 2 minutes: a white precipitate is formed, and an amine odor is perceptible when the mixture cools. Decant the supernatant, and add to the precipitate 3 mL of 3 N hydrochloric acid: effervescence is produced.
C:
A solution (1 in 20) responds to the tests for
Chloride  191
191
.
D:
To a solution of 100 mg in 1 mL of water add 3 mL of gold chloride solution (1 in 10): a precipitate of yellow crystals of the aurichloride is formed. The precipitate, upon recrystallization from about 5 mL of hot water, separates in glistening scale-like crystals which, after drying at 105
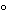
for 1 hour, melt between 183
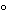
and 185
.
Loss on drying  731
731 —
—
Dry about 1 g, accurately weighed, at 105
for 2 hours: it loses not more than 2.0% of its weight.
Ordinary impurities  466
466 —
—
Test solution:
a mixture of methanol and water (4:1).
Standard solution:
a mixture of methanol and water (4:1).
Eluant:
alcohol.
Visualization:
16.
Assay—
Dissolve about 400 mg of Carbachol, accurately weighed, in a mixture of 10 mL of glacial acetic acid and 10 mL of
mercuric acetate TS. Add 2 drops of
crystal violet TS, and titrate with 0.1 N perchloric acid VS. Perform a blank determination, and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 18.27 mg of C
6H
15ClN
2O
2.